Industrial sector has largely been dependent on exhibitions and trade shows which created sector specific physical ecosystems to…
Ever since Marseille Finson won the Nobel Prize in 1910, for using UV rays to treat bacterial infections and diseases, there have been significant developments in deploying UV rays for a multitude of purposes. From killing disease causing bacteria to curing applications, UV lamps have come a long way.
This blog post is the introductory part of a blog series that covers the basics of UV curing process- as explained by Eric Nelson- Application Manager at Heraeus Noblelight America, in an informative tech-talk with our founder and technology evangelist, Ankit Singhal.
To start with, we have curated this blog around the physics of UV rays and the definition of UV curing. The next blogs would go deeper into the process and its applications.
UV rays in the Electromagnetic Spectrum
To understand the Physics of Ultra violet rays, let us have a look into the electromagnetic spectrum below. In the electromagnetic spectrum, the UV lies in the range 100-400 nm- typically between the visible light and the x-rays.
“Starting with the lower end, we see x-rays- these are lower wavelength energy particles traveling through air, then as you move up there’s UV and visible rays and then at very high level wavelength Infra-red rays”, explains Eric.
“ We usually say the UV falls between 200- 425nm. However, it actually falls a bit lower at 100 nm, and falls in the vacuum region.” Says Eric. The region between 100 nm to 200 nm is referred to as Vacuum UV or VUV.
Eric further explains “This region is actually extremely energetic, and is absorbed by oxygen in the air, so UV cannot transmit to Earth below 200 nm. However, in an inert (non-oxygen) environment you can get lower wavelength UV rays.”
The UV rays are further distributed through lower and higher energy levels, according to the wavelength range,
Eric explains this using the example of Visible light where you see a rainbow of colors ranging from violet at the lower end of the spectrum up until red to the higher end of the spectrum.
UV rays are also distributed in a similar fashion, through different energy zones, from VUV to UV-A, UV-B, and UV-C.
Applications of UV rays
There are a multitude of uses for UV light for various industrial verticals. Each of the UV zones have different energy levels, and hence are used for different applications. Here are some of the significant applications for the four major UV zones.
- UVA This UV zone lies between 315- 400 nm in the spectroscope. The photon energy levels for this range is 3.3 to 4.0 eV and lies closest to the visible rays. UVA is widely used for UV curing applications, which is covered in detail in the later part of the article. Other common uses of UVA include tanning, backlighting, light therapy, spectroscopy, authentic identification and much more.
- UVB This UV zone lies between 280- 315 nm in the spectroscope, with energy levels in the range 4.0 to 4.4 eV. It lies between UVA and UVC zones. UVB is also commonly used for UV curing applications, along with significant uses in healthcare. In medicine UV-B plays a key role in diagnosing and treating skin conditions. “If someone has eczema or any kind of skin damage, it could theoretically be treated using UV -B energy- there are different companies that specifically make UV lamps for this use. “ explains Eric.
- UVC This UV zone lies in the range 200-280 nm with energy levels in the range 4.4 to 6.2 eV. It lies between VUV and UVB zones. UVC is also used for UV curing applications, but is widely known for its disinfection uses. “UV C is used for disenfication applications or what we call- environmental application, which is using UV energy to get rid of different types of microbes, and different viruses.:, explains Eric. The use of UV for disinfection was discovered way back in the late 1800s via sunlight. By the 1930s, it was a widely accepted science and UV lamps were used to treat measles in classrooms or mounting lamps up in the air, explains Eric. However, UV C for disinfection has gained tremendous popularity in the recent COVID time.
- VUV The next zone has the highest energy of them all with the lowest wavelength range of 100-200nm. Eric describes how the high energy of VUV affects its chemistry and human life. “Owing to the high energy, VUV gets absorbed into the air, as it breaks the oxygen molecules into ozone in the air. The ozone layer is highly unstable and potentially dangerous to humans.” “However, ozone does have benefits for advanced oxidation disinfection reactions”, He adds. Typically, VUV is popularly used to create Ozone and for high-speed UV curing applications. The curing application can take place only in a non-oxygen or inert environment like in an Argon or Nitrogen atmosphere, which enables the UV transmission to a surface. Since a vacuum or a void is required to transmit this UV ray, it is named as vacuum UV. The low wavelength makes it highly energetic enough to create stronger reactions to different materials, and used in different cleaning and oxidation processes. The vacuum UV finds applications where there is a need for high speed industrial coating, like mass produced anti-fog coating eyeglasses or lab goggles.
Potential hazards of UV Overexposure
Prolonged exposure to UV rays of any wavelength can prove to be hazardous to humans. It could be as simple as a red rash, or sun burn marks and as severe as skin cancer. As the wavelength gets shorter, the energy increases and so does its hazardous effect.
Therefore, UVA is least hazardous, while UVC has the highest. VUV has little or no human contact in a normal atmosphere.But, in an inert environment where VUV transmits, it can have severe effects on human health like blindness, redness of skin, and even cancer.
What are UV Lamps?
UV lamps produce UV light that create photochemical reactions to cure adhesives, coatings, thin films, and much more. The wavelength plays a vital role in carrying out chemical reactions that produce the desired output to suit various applications. These are a cost-effective alternative to heat-cured inks and coatings.
UV lamps are also designed differently for different applications. Each application requires UV rays of different wavelengths to suit a particular industrial purpose.
UV Lamps for environment applications For example, UV lamps that produce UV of lower wavelength are widely used for different environment applications, like
- UV lamps for disinfection
- Surface disinfection Here, UV lamps are used to disinfect surfaces, like the inside of a yogurt container, different surfaces of milk cartons, packaging material etc, Eric explains. “Shining UV light on the surface of packaging materials increases the shelf life by one or two weeks”, he adds.
- Water disinfection Another common application for UV lamps for surface disinfection is in water treatment. Here the UV lamp is immersed in water, so it can break down the microbes and clear the water.
- Air disinfection UV Lamps are installed in various air treatment systems. The idea here is to inactivate the microbes in the air like viruses that cause illness through the UV light radiation.
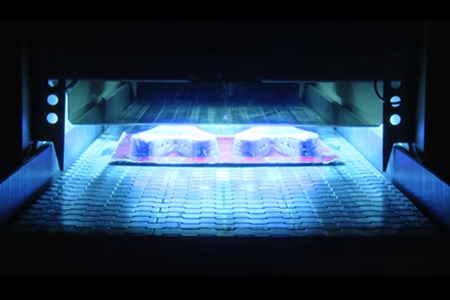
UV Lamps for Curing Applications
Curing is an industrial process where chemical reactions initiate polymerization, in a way that hardens the exposed surface.
A close look into the UV rays application gives an interesting conclusion- UV rays from any spectral zone are capable of UV curing application.
UV curing uses specially designed UV lamps that transmit the rays on a surface to initiate chemical reaction and create solid polymers.
Eric beautifully explains the process through an example.
“You could form a plastic through the traditional thermal route, where you take hot melt of different plastics and dry it out, and it becomes a hardened structure. Or you could shine a UV lamp that initiates a chemical reaction that causes a chain reaction, that causes the material to cross link and become more functional and harder.”
Common Industrial Applications of UV Curing
UV Curing finds applications in almost every industry for:
- Improving throughput,
- Decreasing energy usage, and
- Increasing the reliability of the curing process.
Some of the common industrial applications of UV curing are:
- Packaging- To print labels on each package.
- Flooring and Piping-
- To add simulated coating to the floor
- To add corrosion resistant pipe coating
- Automobile- To add anti-scratch, anti resistant coating on automobile parts.
- Medical- To bond different medical parts system
- Electronics-
- Potting- Coating the surface of the circuit board to prevent dust and moisture accumulation.
- Fiber Optic cables and Semiconductor manufactured using UV light.
Apart from the coating and adhesive applications, UV curing is also used to create thin films for different purposes at high speed.
With this, we come to the end of the first part of the tech- blog series on UV curing applications.
The next two articles will go deep into the UV curing process, uv system reflectors and other relevant technological terms and processes. Follow the UV Curing ecosystem here to get instant notifications, and also know when we release the next part!